Large Volume Parenteral (LVP) Solutions
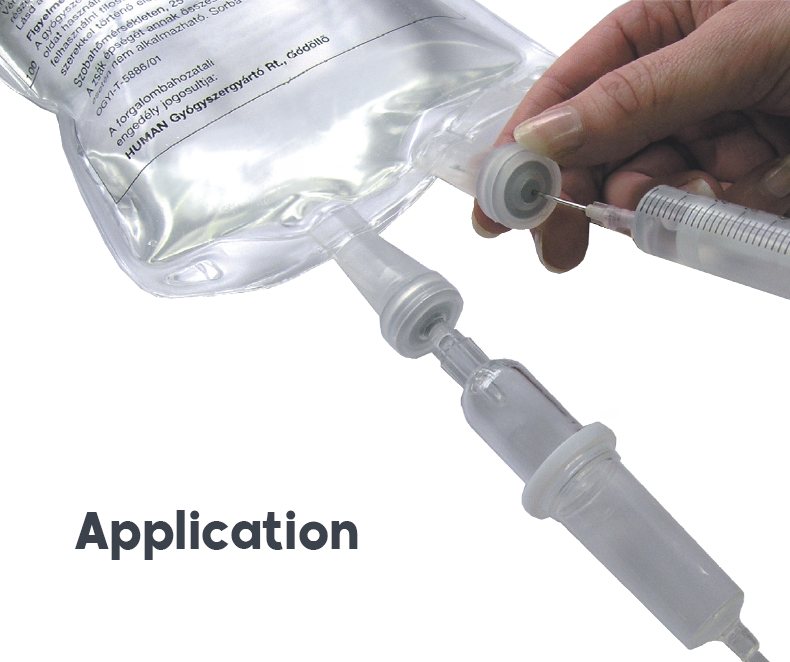 Annual production of several million units and cost optimization are some of the reasons that drive Alison to internalize the production of its intravenous products with industrial tools such as Form Fill Seal (FFS). The FFS machines combine all steps in the packaging process of an intravenous product: manufacturing of the primary packaging, printing, filling, insertion of connectors, sealing, and closing of the tubes. The process also includes control phases that verify the final volume according to weight, as well as detecting leaks in the vacuum chamber (pressure decay testers) or by pressure, and testing the solidity of the bag under high tension.
Annual production of several million units and cost optimization are some of the reasons that drive Alison to internalize the production of its intravenous products with industrial tools such as Form Fill Seal (FFS). The FFS machines combine all steps in the packaging process of an intravenous product: manufacturing of the primary packaging, printing, filling, insertion of connectors, sealing, and closing of the tubes. The process also includes control phases that verify the final volume according to weight, as well as detecting leaks in the vacuum chamber (pressure decay testers) or by pressure, and testing the solidity of the bag under high tension.
After filling and sealing, the bags are placed on trays and enter the autoclave sterilization chambers. FFS systems are also highly flexible. They can be customized to allow the manufacturer different packaging formats, such as single or multiple volume compartments with peelable seals, tubes, etc. The films used must meet numerous requirements, including chemical inertia to avoid interactions between the container and the contents, and specific barrier properties depending on the product. These features ensure our IV bags are compatible with regulatory requirements and industrial tools on the market.
